What is Soap?
- Soaps are sodium or potassium salt of fatty acids that contains 12 to 18 carbon atoms per molecule.
- Soaps are cleansing agents made from animal fats or vegetable oil by saponification.
O
||
CH3-CH2-CH2- CH2-CH2-CH2-CH2- CH2-CH2- CH2-CH2- CH2-
CH2- CH2-CH2- C-O− Na+
Non-polar hydrocarbon chain ionic end.(Soluble in non polar substances) (Soluble in water)
The History of Soap Manufacturing
- Soap have been used for more than 3000 years. It was recorded that the Babylonians were making soaps around 2800 B.C.
- The ‘Purifying Oils’ were recorded on Hebrew tablets in 4000 B.C.
- In ancients time, soap made from ashes of plants which contain sodium carbonate and potassium carbonate. The ashes were boiled with lime (calcium oxide) to produce caustic potash (potassium hydroxide). Caustic potash is then boiled with the animal fats to produce soap.
a) boiled
Ash + Lime -------> Caustic Potash
(K2CO3) (CaO) (KOH)
b) boiled
Caustic Potash + Animal Fats ------->Soap
- In 1861, the Belgian Chemist Ernest Solvay (1838-1922) discovered the process to make soda (sodium carbonate) from common salt (sodium chloride) and calcium carbonate.
- This process is known as the Solvay Process which produces sodium carbonate cheaply for industrial use. Sodium carbonate (often called soda or soda ash) is used for making glass, soaps and detergents.
- Michel Chevreul (1786-1889), a French chemist, was noted for his research in the composition of animal fats is composed of fatty acids and glycerol. This discovery contributed to the rapid development of the soap and candle industry.
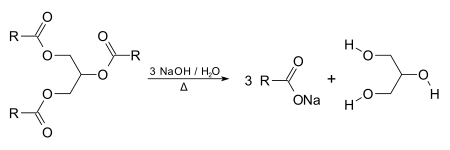
1. Soap is a cleansing agents produced by the reaction between sodium hydroxide and potassium hydroxide with animal fats or vegetable oils. This reaction is known as saponification.
2. Fats and vegetable oils are large, naturally occurring ester molecules. When fats or oils are boiled with concentrated alkalis, such as sodium hydroxide, saponification occurs and the ester molecules are broken down into soap and glycerol.
Fats or vegetable oils + concentrated alkalis -----> soap +glycerol
3. Saponification is the alkaline hydrolysis of ester using alkali solutions. From thechemist aspect, soaps are sodium salts or potassium salts of long chaincarboxylic acids (with 12 to 18 carbon atoms per molecule).
4. Some examples of soaps are shown below.
- Sodium palmitate, C15H31COONa
- Sodium oleate, C17H33COONa
- Sodium stearate, C17H35COONa
Additives such as perfume, colouring matter and sometimes antiseptics are added to soaps to enhance their marketability.
5. Glyceryl tristearates are naturally occurring esters commonly found in animal fats and vegetable oils. When the ester is boiled with concentrated sodium hydroxide solution, saponification (alkaline hydrolysis) occurs and mixture of sodiumstearate (soap) and glycerol is obtained.
6. The soap produced can be precipitated by adding common salt (sodium chloride)to the reaction mixture.
7. The sodium chloride added reduced the solubility of soap in water. As a result,precipitation of soap occurs.
8. The properties of soap depend on :
- The type of alkali used for saponification
- The type of animal fats or vegetable oils used.
10. Animal fats (tallow) from cows and vegetable oils (such as palm oil or olive oil) are used for making soap.

The Cleansing Action of Soap / Detergent
1.The cleansing action of soap or detergent depends on their chemical bonding and structures.
- The ionic ‘head’(negatively charged)is soluble in water (hydrophilic) but insoluble in oily layer.
- The long hydrocarbon ‘tail’(neutral)is insoluble in water (hydrophobic) but soluble in oily layer.
2.Oil cannot be washed away from clothing with water because oil (a covalent molecule is insoluble in water.
3.Lifting greasy dirt from the surface cloth. When soap or detergent is added to the dirty surface of a piece of cloth covered with a layer of oil or grease.
- The negatively charged ‘head’ (hydrophilic) of soap ions or detergent ions dissolves in water.
- The hydrocarbon ‘tail’ (hydrophobic) of soap or detergent ions dissolves in the layer of grease
4.I the water is agitated slightly, the grease begins to be lifted off the surface. This cause by the forces of attraction between the water molecules and the negatively charged heads.
 |
| Cleansing Action of Soap |
6.Emulsifying dirt in water
- Soaps and detergents can act as emulsifying agents to emulsify oils and grease.
- The process of emulsification breaks large drops of grease into smaller droplets that floats in water. The greasy droplets repel on another because they carry the same charge. As a result, the grease is suspended in the solution
- When the cloth is rinsed with the water, the droplet will be carried away.
- The cleaning process become more efficient in the water containing the soap or detergent solution is stirred.
The Effectiveness of Soaps and Detergents as Cleansing Agents
Advantages of Soaps
- Soaps are effective cleansing agents in soft water, that is water does not contain Mg2+and Ca2+ ions.
- Soaps do not cause pollution problems to the environment. This is because soaps are made from chemical found in animals and plants. This means that soaps are biodegradable, that is they can be composed by the action of bacteria.
Disadvantages of Soaps
- Soaps are ineffective in hard water, that is, water that contains magnesium and calcium salts.
- In hard water, soaps will react with Mg2+and thus, soaps do not lather in hard water.
- Scum is grey solid that is insoluble in water. It consists of magnesium stearate and calcium stearate.
- Soaps are not also effective in acidic water, for example rainwater containing dissolves acids. H+ ions from acids will react with soap ions to produce carboxylicacids molecular size that are insoluble in water.
- Stearic acids and other carboxylic acids do not act as cleansing agents because they exist mainly as molecules and do not anionic hydrophilic ends (’head’) that dissolves in water.
Advantages of Detergents
- Detergents are cleansing agents that are effective in soft water as well as hard water. This is because detergents do not form scum with Mg+and Ca2+ions found in hard water.
- The detergents ions (R –O – SO3-and R – SO3-)react with Mg+and Ca2+ions in hard water. However, the magnesium salts and calcium salts which are formed are soluble in water. Hence, the scum is not formed and the detergents are still active in hard water and lathers easily.
- Detergents are synthetic cleansing agents. This means that the structure of the hydrocarbon chain can be modified to produce detergents with specific properties.Nowadays, different types of detergents have been synthesised for specific uses such as shampoos and dish cleaner.
- Furthermore, detergents are also effective in acidic water because H+ ion is acidic water do not combined with detergents ions.
Disadvantages of Detergents
- Most detergents have branched hydrocarbon chains and are non-biodegradable,that is, they cannot decomposed by bacteria. As a result, non-bio degradable detergents cause water pollution.
- Phosphates in detergents act as fertilizers and promote the growth of water plants and algae. When the plants die and decay, they will used up the oxygen dissolves in water. This will decrease the oxygen content in water and kill fishes and other aquatic lives.
- Detergents produce a lot of foam in water. The layer of foam that covers the water surface will prevents oxygen from dissolving in water. This condition will cause fish and other aquatic life ti die from oxygen starvation.
- Additives such as sodium hydrochlorite (bleaching agents) releases chlorine gasin water that is acidic. Chlorine gas is highly toxic and kills aquatic life.
*ADDITIONAL INFORMATION
Soap Preparation Processes
There is three ways to make soap. One of them is Cold Process.
The video below will explain how to make a soap using this process.
Even in the cold-soap making process, some heat is usually required for the process. The temperature is usually raised sufficiently to ensure complete melting of the fat being used. The batch may be kept warm for some time after mixing to ensure that the alkali (hydroxide) is completely used up. This soap is safe to use after approximately 12–48 hours, but is not at its peak quality for use for several weeks.
Cold-process soap making requires exact measurements of lye and fat amounts and computing their ratio, usingsaponification charts to ensure that the finished product does not contain any excess hydroxide or too much free unreacted fat.
A cold-process soap maker first looks up the saponification value of the fats being used on a saponification chart. This value is used to calculate the appropriate amount of lye. Excess unreacted lye in the soap will result in a very high pH and can burn or irritate skin. Not enough lye, and the soap is greasy. Most soap makers formulate their recipes with a 4–10% deficit of lye so that all of the lye is converted and that excess fat is left for skin conditioning benefits.
 |
| Saponification of a triglyceride with sodium hydroxide. |
The lye is dissolved in water. Then oils are heated, or melted if they are solid at room temperature. Once the oils are liquified and the lye is fully dissolved in water, they are combined. This lye-fat mixture is mixed until the two phases (oils and water) are fully emulsified. Emulsification is most easily identified visually when the soap exhibits some level of "trace", which is the thickening of the mixture. (Modern-day amateur soap makers often use a stick blender to speed this process). There are varying levels of trace. Depending on how additives will affect trace, they may be added at light trace, medium trace, or heavy trace. After much stirring, the mixture turns to the consistency of a thin pudding. "Trace" corresponds roughly to viscosity. Essential oils and fragrance oils can be added with the initial soaping oils, but solid additives such as botanicals, herbs, oatmeal, or other additives are most commonly added at light trace, just as the mixture starts to thicken.
The batch is then poured into moulds, kept warm with towels or blankets, and left to continue saponification for 12 to 48 hours. (Milk soaps or other soaps with sugars added are the exception. They typically do not require insulation, as the presence of sugar increases the speed of the reaction and thus the production of heat.) During this time, it is normal for the soap to go through a "gel phase," wherein the opaque soap will turn somewhat transparent for several hours, before once again turning opaque.
After the insulation period, the soap is firm enough to be removed from the mould and cut into bars. At this time, it is safe to use the soap, since saponification is in essence complete. However, cold-process soaps are typically cured and hardened on a drying rack for 2–6 weeks before use. During this cure period, trace amounts of residual lye is consumed by saponification and excess water evaporates.Even in the cold-soap making process, some heat is usually required for the process. The temperature is usually raised sufficiently to ensure complete melting of the fat being used. The batch may be kept warm for some time after mixing to ensure that the alkali (hydroxide) is completely used up. This soap is safe to use after approximately 12–48 hours, but is not at its peak quality for use for several weeks.
Hot-processed soaps are created by encouraging the saponification reaction by adding heat to the reaction. Thisspeeds the reaction. Unlike cold-processed soap, in hot-process soaping the oils are completely saponified by the end of the handling period, whereas with cold pour soap the bulk of the saponification happens after the oils and lye solution emulsification is poured into moulds.
The third, Purification and finishing
The second one is Hot Process
| Hot Process Vegan Soap |
Hot-processed soaps are created by encouraging the saponification reaction by adding heat to the reaction. Thisspeeds the reaction. Unlike cold-processed soap, in hot-process soaping the oils are completely saponified by the end of the handling period, whereas with cold pour soap the bulk of the saponification happens after the oils and lye solution emulsification is poured into moulds.
In the hot-process, the hydroxide and the fat are heated and mixed together 80–100 °C, a little below boiling point, until saponification is complete, which, before modern scientific equipment, the soapmaker determined by taste (the sharp, distinctive taste of the hydroxide disappears after it is saponified) or by eye; the experienced eye can tell when gel stage and full saponification has occurred. Beginners can find this information through research and classes. Tasting soap for readiness is not recommended, as sodium and potassium hydroxides, when not saponified, are highly caustic.
An advantage of the fully boiled hot process in soap making is that the exact amount of hydroxide required need not be known with great accuracy. They originated when the purity of the alkali hydroxides were unreliable, as these processes can use even naturally found alkalis such as wood ashes and potash deposits. In the fully boiled process, the mix is actually boiled (100C+), and, after saponification has occurred, the "neat soap" is precipitated from the solution by adding common salt, and the excess liquid drained off. This excess liquid carries away with it much of the impurities and color compounds in the fat, to leave a purer, whiter soap, and with practically all the glycerine removed. The hot, soft soap is then pumped into a mould. The spent hydroxide solution is processed for recovery of glycerine.
A generic bar of soap, after purification and finishing.
In the fully boiled process on factory scale, the soap is further purified to remove any excess sodium hydroxide, glycerol, and other impurities, colour compounds, etc. These components are removed by boiling the crude soap curds in water and then precipitating the soap with salt.
At this stage, the soap still contains too much water, which has to be removed. This was traditionally done on a chill rolls, which produced the soap flakes commonly used in the 1940s and 1950s. This process was superseded by spray dryers and then by vacuum dryers.
The dry soap (approximately 6–12% moisture) is then compacted into small pellets or noodles. These pellets/noodles are now ready for soap finishing, the process of converting raw soap pellets into a saleable product, usually bars.
Soap pellets are combined with fragrances and other materials and blended to homogeneity in an amalgamator (mixer). The mass is then discharged from the mixer into a refiner, which, by means of an auger, forces the soap through a fine wire screen. From the refiner, the soap passes over a roller mill (French milling or hard milling) in a manner similar to calendering paper or plastic or to making chocolate liquor. The soap is then passed through one or more additional refiners to further plasticize the soap mass. Immediately before extrusion, the mass is passed through a vacuum chamber to remove any trapped air. It is then extruded into a long log or blank, cut to convenient lengths, passed through a metal detector, and then stamped into shape in refrigerated tools. The pressed bars are packaged in many ways.










